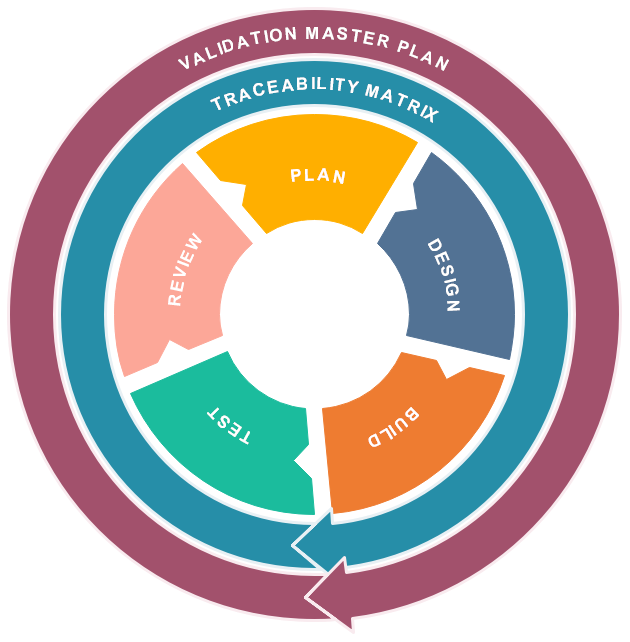
Comprehensive Computer System Validation Services
Our expert team delivers end-to-end validation services following GAMP 5 methodology and regulatory requirements
Validation Planning & Strategy
- Validation Master Planning
- Risk Assessment
- Requirements Specification
- Validation Strategy Development
Validation Execution
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
Compliance & Maintenance
- Periodic Review
- Change Management
- Data Integrity Assessment
- Compliance Monitoring
Specialized Systems
- LIMS Validation
- ERP System Validation
- CTMS Validation
- Cloud System Validation
Training & Support
- CSV Methodology Training
- Regulatory Requirements
- Documentation Best Practices
- Ongoing Support
Audit & Assessment
- Vendor Audits
- System Assessment
- Gap Analysis
- Remediation Planning
Help & Guidance
Answers to Common Questions
Meet Our Team
Experts Driving Your Success
Shahrzad Eghbali
Manager
Behzad Sanie
Manager
Michael Harris
Manager
What Our Clients Say